Common class of antidepressants may help with…Covid?
Reminder: SSRIs are not ready for prime-time Covid treatment and there is only one promising exploratory study. This should not be routine clinical care at this time.
We are still seeing a lot of patients with Covid in the Emergency Department and most can be discharged home. Over the last 8 months, care pathways have advanced our ability to follow these patients (telemedicine, pulse oximeters, vital sign monitoring devices) but treatment options for the discharged mild/moderate patient have been limited. The monoclonal antibodies have now opened up some options but the criteria for use are strict and the data is limited. Are there other potential, evidence-based, treatment modalities?
Since the Spring of 2020, thousands of articles have either been published or submitted claiming a potential therapeutic treatment modality for Covid-19. The problem with many of these studies is that they are usually not very well designed clinical trials. Often the studies are observational in nature, leading to numerous confounders. Extra attention should be given to studies that are randomized and conducted prospectively in the era of Covid (not an easy task). Double-blind, placebo controlled trials during this pandemic (as groups rush to meet clinical gaps and struggle with safe ways to deliver drugs and conduct research visits) are a rarity. However, an 11/12 article by Eric Lenze, MD and colleagues published in JAMA pulled off a small double-blind, placebo controlled RCT which tested a novel therapeutic in a very important space — Covid patients discharged home with mild/moderate Covid symptoms. Most of the available Covid treatments are for hospitalized patients (remdesivir, steroids) requiring oxygen support. In November, some monoclonal antibodies (Eli-Lilly’s bamlanivimab and Regeneron’s cocktail infusion of casirivimab and imdevimab) were given an emergency use authorization by the FDA for use outside of the hospital for mild/moderate Covid. Interestingly, both the monoclonal antibodies and the therapeutic studied in the Lenze et al.,. trial considered process outcomes — hospitalization/deterioration.
So, what is the therapeutic studied in the Lenze et al., RCT? A theme during this pandemic is that drugs created for other uses have been studied as possible repurposed tools to fight Covid infections — some successfully (Remdesivir, the “Mabs”, decadron, maybe one Jax inhibitor) and some, not so much (hydroxychloroquine, multiple other JAX inhibitors, multiple anti-virals, IL-6 inhibitors). Well, why not try selective serotonin re-uptake inhibitor antidepressants (SSRIs)?
What is the potential mechanism here? Essentially, blockade of the inflammatory cascade through disruption of cytokine production. Turns out that SSRIs, especially fluvoxamine, bind at a cellular site involved in cytokine production and were studies in sepsis models to decrease a run away inflammatory response. Editor (me) note here though — we aren’t exactly using a lot of SSRIs to treat sepsis and this concept of just blocking the inflammatory response rarely works in translation from pharmaceutical concept to clinical outcomes (um, Xigris anyone?)
Anyway, the hypothesis was ok (that using an SSRI with ability to disrupt cytokine production may slow the inflammatory cascade that worsens outcomes in the second pathological phase of the virus), the study design itself was pretty good for a small pilot study (again, this drug is not at all ready for primetime but should earn itself a larger funded clinical study given these preliminary results).
The study was small — just 152 adults but they were randomized to receive fluoxamine or placebo and neither the researchers or the patients new which patients were on which agent (double-blind). The whole trial was also conducted remotely after identification, often in the emergency department. For those of us working in Covid research, consent processes, drug delivery and study visits have been logistical challenges thus the methods section is worth a read. Patients were consented electronically, meds, a pulse oximeter, a blood pressure monitor and a thermomemter were all delivered to the patient. Researchers conducted phone calls with participants to assess symptoms.
During the phone calls, researchers asked patients if they had dyspnea (using a scale), whether they had been hospitalized or if there pulse oximetry reading had dipped under 92%. Together, any of these three findings were considered “clinical deterioration”, the primary endpoint.
The particpants were followed for 15 days and, a little odd for an exploratory study like this, researchers allowed participants to receive open label fluoxamine after the 15 days if they desired to take the medication. In addition, following the pre-specified 15 day follow up endpoint, a 30 day survey was also performed asking if patients had been hospitalized.
Emergency medicine studies often utilize clinical or process driven endpoints, however, convincing the rest of the house of medicine to use such endpoints is not always easy and many studies have had statistically significant results with unclear clinical meaning. The use of process and patient centered flow measures marks an excellent way to consider whether a novel treatment strategy is useful in an evolving pandemic that has threatened hospital capacity and made throughput issues front page problems in the American Health Care System.
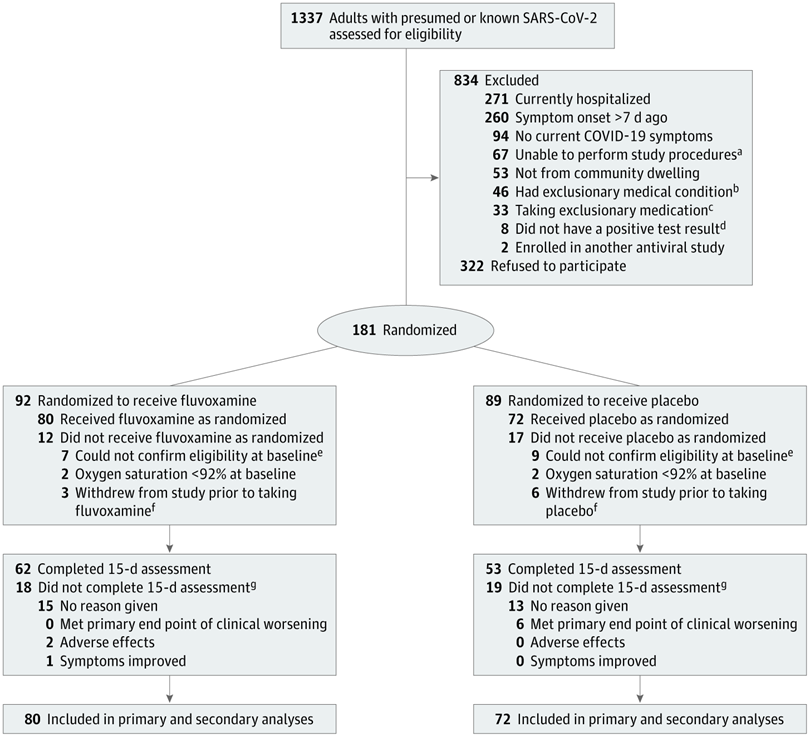
Recruitment took some time (April to August, 2020) as many patients were excluded (See Figure 1 below) and others declined participation. Ultimately 181 patients were randomized but an about even number in both groups did not follow up at 15 days, leaving 80 particpants in the fluvoxamine arm and 72 in the placebo group.
The results are very intruiging. ZERO patients in the fluvoxamine group had clinical deterioration while 6 of the 72 in the placebo group deteriorated (8.3%, 95% CI[1.8%-16.4%]). 4 of those 6 patients were hospitalized. None of the patients in the fluvoxamine arm were hospitalized (although one of the fluvoxamine patients was later hospitalized for, what was described as a post-Covid headache between 16 and 30 days). The p value was < 0.009 so these results are not likely due to chance and, even if the actual results are closer to the bottom of the CI range, at least 1 hospitalization was likely prevented (potentially stronger data than the monoclonal antibody treatments that have both received EUAs in which at least 20 patients likely need to receive antibody infusion to prevent one hospitalization).
While this trial is small, keep in mind that Eli-Lilly received an EUA for bamlanivimab based on only 101 patients in the 700mg arm and 143 pateints in the placebo arm, published in the NEJM. Having said that, monoclonal antibodies have been studied for a longer period of time and propose a specific therapeutic pathway that itself is efficacious — i.e. an agent that attacks an invading particles critical receptor domain (in this case, the Spike Protein on the surface of Sars-CoV-2) — while this is a novel pathway and a novel agent (or repurposed agent in this case).
There have been a LOT of attempts to repurpose drugs to find a space in the Covid landscape but the underlying pharmaceutical/therapeutic hypotheses have been questionable and the methods rushed given need to simultaneously attempt clinical use. This small study advances Covid research methodology and demonstrates that research with Covid+ patients discharged from an ED setting can and should be done and that, with a solid pharmacotherapeuic hypothesis, some repurposing of older/other agents may be reasonable.
Take Home Point:
This is an interesting study that shows potential for repurposing SSRIs for the treatment of Covid. It is important to remember that SSRIs are not ready for prime-time Covid treatment and there is only one promising exploratory study. This should not be routine clinical care at this time.
References:
2. Covid, the Spike Protein, & Monoclonal Antibody Treatments
3. Lilly Announces withdrawal of Xirgris Following Recent Clinical Trial Results.
4. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients …www.nejm.org › doi › full › NEJMoa2029849
About the Author:
Jason Wilson, MD is a core faculty member for USF EM and, as research director, coordinates multiple Covid research studies across TGH/USF.